The effective nuclear charge on the atom will affect the energy of the valance electrons. Hence, the reactivity of an atom arises from the existence of unpaired electrons in the valence shell. Expert educator Michael R. From University of Miami explains The reactivity of an atom arises from. See the full lesson video for free here: https://www.nu. The reaction of the iron(IV)‐oxo complexes that is of most interest is hydrogen atom transfer with hydrocarbons, which we have studied with 1‐methylcyclohexene (che, Figure 1 c). After adding the hydrocarbon to the vial C, we can monitor the HAT reaction proceeding in solution before ESI‐MS detection.
- Information About Atoms
- The Reactivity Of An Atom Arises From
- What Determines The Reactivity Of An Atom
What does the reactivity of an atom arise from?
1 Answer
Explanation:

Information About Atoms
The second law of thermodynamics says that everything in the universe moves from order to disorder.
A corollary of the second law is that everything moves to state of greater stability. Atoms will always move from an unstable structure to a more stable structure.
A atom like Sodium is unstable electronically. Sodium has one valance electron in the outer shell. This is an unstable structure the Sodium atom will react to remove the unstable electron and achieve greater stability. Atoms react chemically to achieve more stable less ordered systems.
An atom like Carbon 14 is unstable in its nucleus. Carbon 14 will react to achieve a more stable nuclear structure. The emission of a beta particle will cause the Carbon 14 to become the more stable Nitrogen 14.
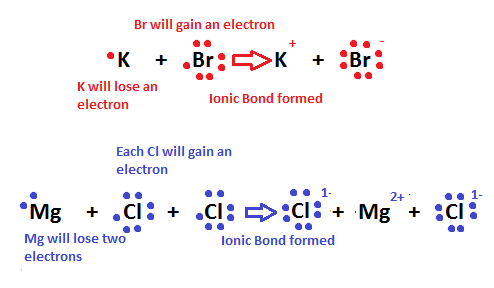

The Reactivity Of An Atom Arises From
Either chemically or nuclear atoms will move to achieve a more stable structure.
What Determines The Reactivity Of An Atom
Related topic
Related questions
