Date Issued: February 19, 2021
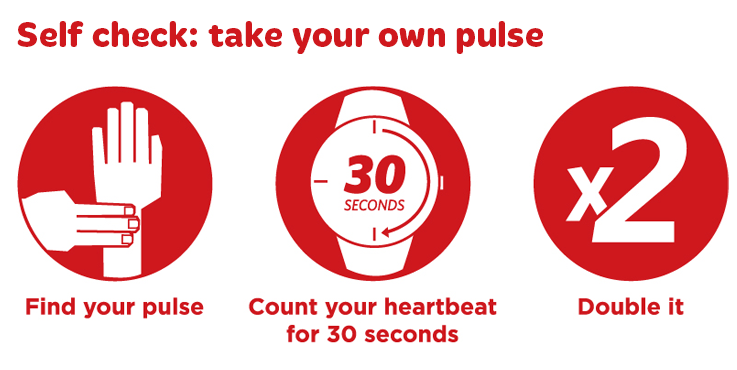
Pulse is now available on IOS. See Instruction. Forgot my password. Click here to see your activities. Forgot my password. Click here to see your activities. Your pulse is your heart rate, or the number of times your heart beats in one minute. You can take your pulse using the radial artery in your wrist or the carotid artery in your neck. Some medical conditions can be a factor in determining the best place for you to take your.
The Coronavirus Disease 2019 (COVID-19) pandemic has caused an increase in the use of pulse oximeters, and a recent report (Sjoding et al.) suggests that the devices may be less accurate in people with dark skin pigmentation. The U.S. Food and Drug Administration (FDA) is informing patients and health care providers that although pulse oximetry is useful for estimating blood oxygen levels, pulse oximeters have limitations and a risk of inaccuracy under certain circumstances that should be considered. Patients with conditions such as COVID-19 who monitor their condition at home should pay attention to all signs and symptoms of their condition and communicate any concerns to their health care provider.
Recommendations for Patients and Caregivers
How to take a reading:
- Follow your health care provider’s recommendations about when and how often to check your oxygen levels.
- Be aware that multiple factors can affect the accuracy of a pulse oximeter reading, such as poor circulation, skin pigmentation, skin thickness, skin temperature, current tobacco use, and use of fingernail polish. To get the best reading from a pulse oximeter:
- Follow the manufacturer’s instructions for use.
- When placing the oximeter on your finger, make sure your hand is warm, relaxed, and held below the level of the heart. Remove any fingernail polish on that finger.
- Sit still and do not move the part of your body where the pulse oximeter is located.
- Wait a few seconds until the reading stops changing and displays one steady number.
- Write down your oxygen levels with the date and time of the reading so you can easily track changes and report these to your health care provider.
How to interpret a reading:
- When taking pulse oximeter measurements, pay attention to whether the oxygen level is lower than earlier measurements, or is decreasing over time. Changes or trends in measurements may be more meaningful than one single measurement. Over the counter products that you can buy at the store or online are not intended for medical purposes.
- Do not rely only on a pulse oximeter to assess your health condition or oxygen level.
- If monitoring oxygen levels at home, pay attention to other signs or symptoms of low oxygen levels, such as:
- Bluish coloring in the face, lips, or nails;
- Shortness of breath, difficulty breathing, or a cough that gets worse;
- Restlessness and discomfort;
- Chest pain or tightness; and
- Fast or racing pulse rate.
- Be aware that some patients with low oxygen levels may not show any or all of these symptoms. Only a health care provider can diagnose a medical condition such as hypoxia (low oxygen levels).
When to contact your health care provider:
Www.mypulsenews.com
- If you are concerned about the pulse oximeter reading, or if your symptoms are serious or getting worse, contact a health care provider.
- If you think you may have COVID-19, contact your health care provider or local health department about getting a diagnostic test for COVID-19. Pulse oximeters cannot be used to diagnose or rule out COVID-19.
For more consumer information on pulse oximeters, see Pulse Oximeters and Oxygen Concentrators: What to Know About At-Home Oxygen Therapy.
Recommendations for Health Care Providers

- Be aware that multiple factors can affect the accuracy of a pulse oximeter reading, such as poor circulation, skin pigmentation, skin thickness, skin temperature, current tobacco use, and use of fingernail polish. Review the information in the sections below to better understand how accuracy is calculated and interpreted.
- Refer to the device labeling or the manufacturer’s website to understand the accuracy of a particular brand of pulse oximeter and sensor. Different brands of pulse oximeters and even different sensors (finger clip versus adhesive) may have a different accuracy level. Pulse oximeters are least accurate when oxygen saturations are less than 80%.
- Consider accuracy limitations when using the pulse oximeter to assist in diagnosis and treatment decisions.
- Use pulse oximeter readings as an estimate of blood oxygen saturation. For example, a pulse oximeter saturation of 90% may represent an arterial blood saturation of 86-94%.
- When possible, make diagnosis and treatment decisions based on trends in pulse oximeter readings over time, rather than absolute thresholds.
Device Description
A pulse oximeter is a device that is usually placed on a fingertip. It uses light beams to estimate the oxygen saturation of the blood and the pulse rate. Oxygen saturation gives information about the amount of oxygen carried in the blood. Adobe photoshop cs6 free download mac full version. The pulse oximeter can estimate the amount of oxygen in the blood without having to draw a blood sample.
Most pulse oximeters show two or three numbers. The most important number, oxygen saturation level, is usually abbreviated SpO2, and is presented as a percentage. The pulse rate (similar to heart rate) is abbreviated PR, and sometimes there is a third number for strength of the signal. Oxygen saturation values are between 95% and 100% for most healthy individuals, but sometimes can be lower in people with lung problems. Oxygen saturation levels are also generally slightly lower for those living at higher altitudes.
There are two categories of pulse oximeters: prescription use and over the counter (OTC).
- Prescription oximeters are reviewed by the FDA, receive 510(k) clearance, and are available only with a prescription. The FDA requires that these pulse oximeters undergo clinical testing to confirm their accuracy. They are most often used in hospitals and doctors’ offices, although they may sometimes be prescribed for home use.
- Over-the-counter (OTC) oximeters are sold directly to consumers in stores or online and include smart phone apps developed for the purpose of estimating oxygen saturation. Use of OTC oximeters has increased as a result of the COVID-19 pandemic. These products are sold as either general wellness or sporting/aviation products that are not intended for medical purposes, so they do not undergo FDA review. OTC oximeters are not cleared by the FDA and should not be used for medical purposes.
For more information on pulse oximeter regulation, see Pulse Oximeters - Premarket Notification Submissions [510(k)s]: Guidance for Industry and Food and Drug Administration Staff.
Interpretation and Limitations of Pulse Oximetry

Pulse oximeters have limitations and a risk of inaccuracy under certain circumstances. In many cases, the level of inaccuracy may be small and not clinically meaningful; however, there is a risk that an inaccurate measurement may result in unrecognized low oxygen saturation levels. Therefore, it is important to understand the limitations of pulse oximetry and how accuracy is calculated and interpreted.
FDA-cleared prescription pulse oximeters are required to have a minimum average (mean) accuracy that is demonstrated by desaturation studies done on healthy patients. This testing compares the pulse oximeter saturation readings to arterial blood gas saturation readings for values between 70-100%. The typical accuracy (reported as Accuracy Root Mean Square or Arms) of recently FDA-cleared pulse oximeters is within 2 to 3% of arterial blood gas values. This generally means that during testing, about 66% of SpO2 values were within 2 or 3% of blood gas values and about 95% of SpO2 values were within 4 to 6% of blood gas values, respectively.
However, real-world accuracy may differ from accuracy in the lab setting. While reported accuracy is an average of all patients in the test sample, there are individual variations among patients. The SpO2 reading should always be considered an estimate of oxygen saturation. For example, if an FDA-cleared pulse oximeter reads 90%, then the true oxygen saturation in the blood is generally between 86-94%. Pulse oximeter accuracy is highest at saturations of 90-100%, intermediate at 80-90%, and lowest below 80%. Due to accuracy limitations at the individual level, SpO2 provides more utility for trends over time instead of absolute thresholds. Additionally, the FDA only reviews the accuracy of prescription use oximeters, not OTC oximeters meant for general wellness or sporting/aviation purposes.
Many patient factors may also affect the accuracy of the measurement. The most current scientific evidence shows that there are some accuracy differences in pulse oximeters between dark and light skin pigmentation; this difference is typically small at saturations above 80% and greater when saturations are less than 80%. In the recently published correspondence by Sjoding, et. al., the authors reported that Black patients had nearly three times the frequency of occult hypoxemia (low oxygen levels in the blood) as detected by blood gas measurements but not detected by pulse oximetry, when compared to White patients. It is important to note that this retrospective study had some limitations. It relied on previously collected health record data from hospital inpatient stays and could not statistically correct for all potentially important confounding factors. However, the FDA agrees that these findings highlight a need to further evaluate and understand the association between skin pigmentation and oximeter accuracy.
My Pulse Rf
All premarket submissions for prescription use oximeters are reviewed by the FDA to ensure that clinical study samples are demographically representative of the U.S. population, as recommended by FDA guidance, Pulse Oximeters - Premarket Notification Submissions [510(k)s]: Guidance for Industry and Food and Drug Administration Staff. As described in this guidance, FDA recommends that every clinical study have participants with a range of skin pigmentations, including at least 2 darkly pigmented participants or 15% of the participant pool, whichever is larger. Although these clinical studies are not statistically powered to detect differences in accuracy between demographic groups, the FDA has continued to review the effects of skin pigmentation on the accuracy of these devices, including data from controlled laboratory studies and data from real world settings.
FDA Actions
The FDA is committed to the continued evaluation of the safety, effectiveness, and availability of medical devices, especially devices in high demand during the COVID-19 pandemic. The FDA is evaluating published literature pertaining to factors that may affect pulse oximeter accuracy and performance, with a focus on literature that evaluates whether products may be less accurate in individuals with darker skin pigmentation. The FDA has been working on additional analysis of premarket data, as well as working with outside stakeholders, including manufacturers and testing laboratories, to analyze additional postmarket data to better understand how different factors including skin pigmentation may affect pulse oximeter accuracy. Based on these findings, the FDA may reassess the content of the pulse oximetry guidance document.
The FDA will keep the public informed if significant new information becomes available.
My Pulse Hurts
Reporting Problems with a Pulse Oximeter
If you think you have a problem with a pulse oximeter, the FDA encourages you to report the problem through the MedWatch Voluntary Reporting Form.
My Pulse Secure
Health care personnel employed by facilities that are subject to the FDA's user facility reporting requirements should follow the reporting procedures established by their facilities.
Questions?
If you have questions, email the Division of Industry and Consumer Education (DICE) at DICE@FDA.HHS.GOV or call 800-638-2041 or 301-796-7100.
- Don't have an account? Sign Up Now!
Security Best Practice and Recommendations:(Updated March 31st, 2021)
Ivanti released a new Ivanti Integrity Checker Tool as well as a security enhancement to augment the current protective measures while further enabling customers to quickly pinpoint unusual activity. For further information on the enhancements, please visit our Quick Start Guide KB44755 and Customer FAQ KB44764.
Ivanti recommends that customers deploy the latest version of Pulse Secure software (9.1R11.1) for their use case and to include password update frequency in their corporate security policy. For more information on security best practices please visit our Technical Bulletin.
Important Information ( April 11th, 2021)
Pulse Secure Host Checker code signing certificate has expired
Please refer to following knowledge article for latest status and remediation KB44781.
Security Advisory Alert (Updated April 19th, 2021)
We recently update the Security Advisory for CVE-20211-22893. Please visit SA44784 for more information.
Contact support or view our FAQs How-To GuidesPassword